DOH studying Israeli saliva test for COVID-19
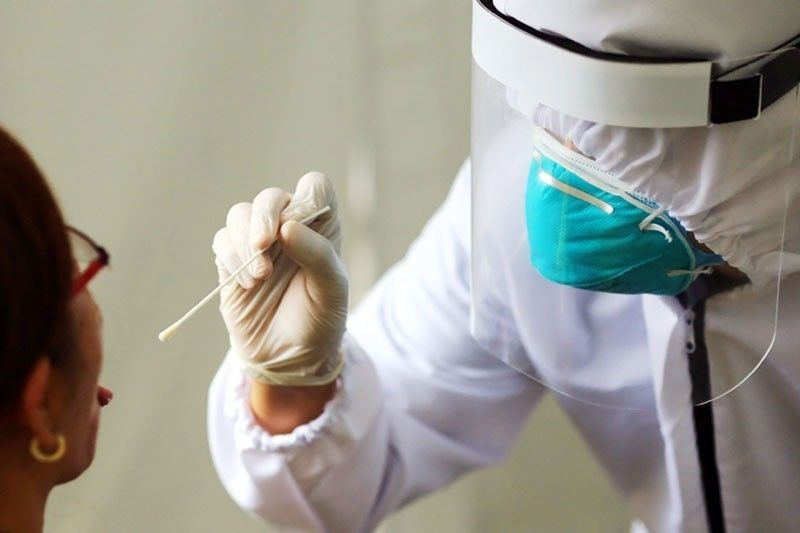
MANILA, Philippines — Israel’s saliva test is now among the test methods for COVID-19 being studied by the Department of Health (DOH), an official said yesterday.
According to DOH Undersecretary Maria Rosario Vergeire, the agency’s laboratory expert panel has been studying the saliva test, which was reported to determine in less than a second if a person is infected with the coronavirus or not.
“I think we got that information around three weeks or one month ago already and our Laboratory Expert Panel is studying the experiences of other countries that are using this method,” she noted.
The experts are then expected to submit a recommendation to DOH.
In using the test kit, patients rinse their mouth with saline wash and spit into a vial.
The saliva will then be examined using a small spectral device which shines light on the samples and analyze the reaction to see if it is consistent with COVID-19.
The trial being done on the test kits is led by Eli Schwartz of the Center for Geographic Medicine and Tropical Diseases at Sheba Medical Center.
Vergeire said it is also being studied if saliva can be collected as samples for testing to detect the presence of SARS-CoV-2.
Currently, the samples being collected in testing a patient using the reverse transcription-polymerase chain reaction (RT-PCR) come from “oropharyngeal.”
“We also use (samples from the) lower respiratory tract if the patient is confined in a hospital. We get this kind of sample,” she added.
The DOH is coming up with omnibus guidelines on the proper use of test methods now available in the market, such as RT-PCR, rapid antibody test kits and rapid antigen.
COVID medicine
The Dangerous Drugs Board (DDB) has issued regulatory guidelines on the importation and sale of a traditional Chinese medicines touted as COVID-19 treatment.
The DDB has issued a resolution prescribing regulatory control requirements for the importation, sale, distribution and prescription of Lianhua Qingwen capsules.
The capsules were earlier approved by the Food and Drug Administration (FDA) as a traditional herbal product as indicated in the Certificate of Product Registration.
It contains plant-based Ephedra and derivative Ephedrine, which is locally classified as a dangerous drug, that need to be regulated by the DDB.
The DDB noted that there is low or negligible risk of abuse due to the “insignificant content” of ephedra in the medicine preparation.
Only 9.14 milligrams of ephedra extract were found during laboratory analysis of the capsule.
Still, DDB said that enforcement agencies are ready to monitor the level of distribution of the product.
It warned the public that online sale and distribution of the capsules are prohibited and may be charged for violations against the Philippine Pharmacy Act or Republic Act 10918.
The DDB said that importers or distributors of the capsules secure a S-5-I license or the license to import drug preparations containing controlled chemicals from the Philippine Drug Enforcement Agency, with each batch accompanied by an import permit.
As for use, Lianhua Qingwen capsules can only be prescribed by a physician with a PDEA S-2 license, even with ordinary prescription in triplicate copies.
The Chinese embassy welcomed the approval, saying that it is used to treat mild to moderate cases of COVID-19 in China and paves the way for traditional Chinese medicine (TCM) into the local market.— Romina Cabrera
- Latest
- Trending



























