Lacking documents, Sputnik V gets stuck in Philippines’ regulatory limbo
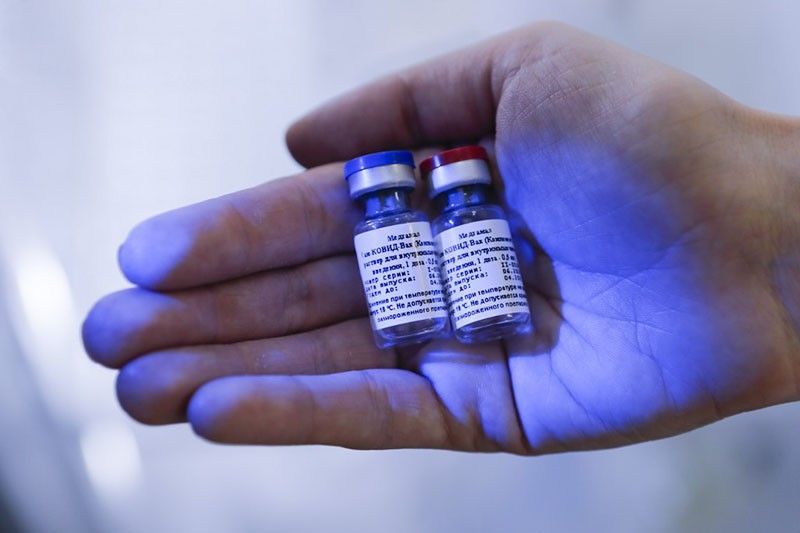
MANILA, Philippines — Russia’s Sputnik V vaccine for COVID-19 is stuck in regulatory limbo in the Philippines as the country’s Food and Drug Administration (FDA) reported Thursday it still lacks some crucial documents to speed up its approval for emergency use.
Among these documents is a Good Manufacturing Practice (GMP) certification, which FDA Director General Eric Domingo explained would assure local regulators of the consistency of the vaccine.
“It assures us that each and every vial that would come out would be identical to those used in the studies and then used somewhere else, and it’s going to be the same quality, and the stability, when it gets here, when it travels, will be maintained,” Domingo told a House panel.
He said the Russian vaccine’s local distributor, Philipine Archipelago International Trading Corp., would have to apply for a GMP certification, after which a team from the FDA would be sent to Russia to inspect the vaccine’s factory.
“If we have that application for the GMP, we can do inspection anytime within the next two weeks. It’s quick, usually the inspection only takes three days,” Domingo said in a mix of English and Filipino.
For its part, the Russian Embassy assured that it would assist the Philippine team who would go to Russia to inspect the vaccine’s factory.
“In this case, I suppose, we will give full visa assistance. We will issue visas as soon as possible,” Vladislav Mongush, the embassy's first secretary, said.
Another crucial document lacking for the speedy approval of Sputnik V for emergency use is an authorization from its manufacturer that would allow Philippine Archipelago International Trading Corp. to sign documents on their behalf.
Domingo said it would be “a lot faster” if the vaccine’s local distributor would be able to sign documents on their behalf.
“We need some documents, of course, to be attested to by the manufacturer, and we know the manufacturer is in Russia and it’s very difficult for some documents to go there and then get here,” he said.
Domingo also said that vaccine experts are still “fine-tuning” their recommendations on the safety and efficacy of Sputnik V, particularly on whether it would be effective for Filipinos as it uses a modified virus to deliver instructions to cells.
He said the Department of Science and Technology is conducting a study whether many Filipinos have already been infected with adenovirus-5, the viral vector used in Sputnik V, which he said may potentially lessen the efficacy of the vaccine.
He also said that the Gamaleya National Center of Epidemiology and Microbiology, which developed the vaccine, submitted Thursday more documents to support their application for emergency use authorization (EUA) and that regulatory officers are currently checking these.
Gamaleya applied last January 6 for an EUA for Sputnik V, which, according to a study published in The Lancet medical journal is 91.6% effective.
Kirill Dmitriev, chief executive officer of the Russian Direct Investment Fund which bankrolled Sputnik V, previously said that Russia is ready to supply COVID-19 vaccines to the Philippines as soon as it secures an EUA.
- Latest
- Trending