FDA allows 2 more hospitals to use Ivermectin
MANILA, Philippines — Two more hospitals were granted compassionate special permits (CSPs) by the Food and Drug Administration (FDA) to use anti-parasitic drug Ivermectin on COVID-19 patients.
According to FDA director general Eric Domingo, they have approved the applications for CSPs of the two unnamed hospitals.
This brings to five the total number of hospitals that are now allowed to access the veterinary drug to combat COVID-19.
Domingo said in an interview that as part of the conditions of the CSPs, the requesting hospitals are required to submit a monthly report to FDA about the patients given Ivermectin.
He added the doctors should also “take full responsibility for the safety and efficacy of the medicine.”
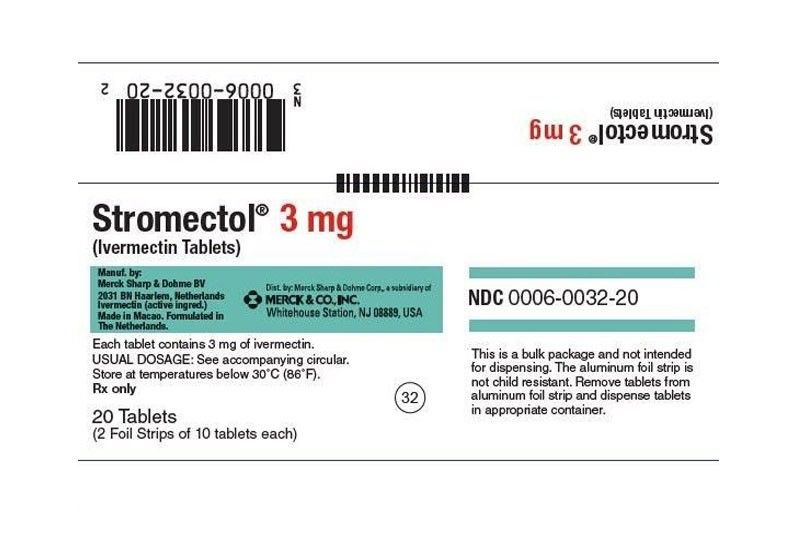
Based on FDA Advisory 2021-0526 dated March15, the registered Ivermectin products in the country for human use are “for topical formulation under prescription use only” – specifically for the treatment of external parasites such as head lice and skin conditions such as rosacea.
The advisory showed that the registered oral and intravenous preparations for Ivermectin are “veterinary products which are approved for use in animals for the prevention of heartworm diseases and treatment of internal and external parasites in certain animal species.”
The FDA had warned that benefits and safety of Ivermectin against COVID-19 have not yet been established.
Health Secretary Francisco Duque III said yesterday he is looking forward to clinical trials to be conducted by the Department of Science on Technology (DOST) on the use of Ivermectin as a treatment for COVID-19.
Duque noted that the DOST has already “laid down human trials for Ivermectin.”
“I’m just waiting for more data. They need to prove the theory during clinical trials,” he said in a television interview.
President Duterte has ordered the DOST to establish the therapeutic effects of Ivermectin on COVID-19 patients through local clinical trials.
He said the “best provider” data on Ivermectin is the World Health Organization, which recently took the position that Ivermectin should not be used outside clinical trial settings.
“We are guided by the WHO advisory on Ivermectin but this is not to prevent us from holding our own clinical trial,” he maintained.
Meanwhile, Albay Rep. Joey Salceda, chairman of the House committee on ways and means, has filed a bill in the House of Representatives encouraging the use of generic medicines or “biosimilar alternatives” to help indigent patients get greater access to cheaper medicines, particularly for COVID-19.
Salceda’s House Bill 9261 (Biosimilars Bill) seeks to open the market to “competitors’ versions of branded drugs” that are cheaper and affordable to indigent patients.
“Biosimilars are basically drugs that are made of the same chemicals and have the same effects as branded expensive drugs. They usually enter the market at a discount, which offers patients and the health care system the potential for savings,” he said.
“Part of what makes branded drugs expensive is that the consumer is paying for the brand. Health care is a matter of life or death, not a matter of one brand being ‘better,’ especially if the chemical composition is practically the same as cheaper drugs,” Salceda explained.
“The people are desperate for cheaper medicine. That’s why you see this hysteria over unsubstantiated cures for COVID-19. That is not good for the people. We still need proven drugs,” he said.
“But, if the proven drugs have less expensive twins, the public should know these alternatives exist,” Salceda added.
“For something as simple as, say, Vitamin C supplements, you look at the generic names and they are practically made of the same chemicals, but the price range can be from 25 centavos to P10 per tablet,” he said.
“Why pay P10 when you get the same effects from the one at 25 cents? This matter is lifesaving for the poor,” he pointed out.
“What this bill does is really help people find cheaper alternatives to the drugs they are prescribed without compromising the integrity of the cure. For matters of life and death, brand is the least of our concerns,” Salceda pointed out. – Delon Porcalla
- Latest
- Trending