FDA grants EUA to Moderna jab
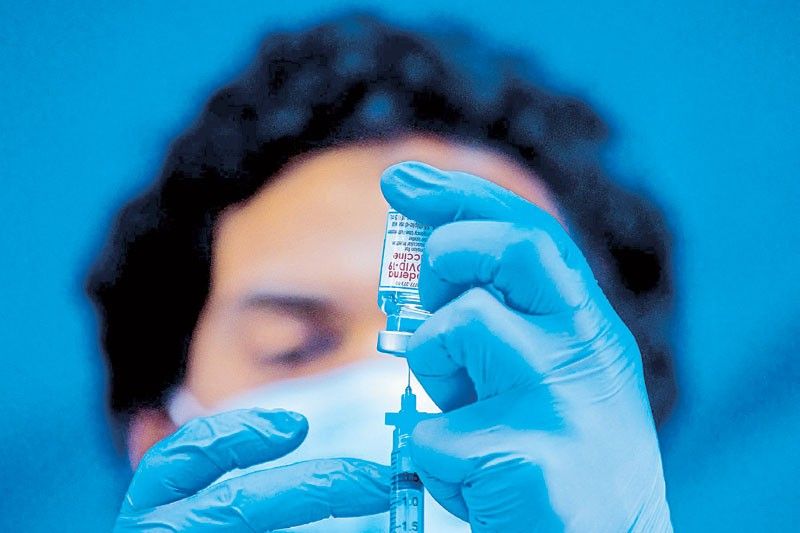
MANILA, Philippines — The Food and Drug Administration (FDA) has approved the use of the vaccine developed by US biotech firm Moderna in the government’s COVID immunization program.
“After a rigorous and thorough review by regulatory and medical experts using currently available published and unpublished data, the FDA is granting emergency use authority for Moderna,” FDA director general Eric Domingo said during the Kapihan sa Manila Bay virtual forum yesterday.
“It has been decided that all conditions for an EUA are present and that the benefit of using the Moderna vaccine outweighs the known and potential risks,” Domingo added.
He said an EUA is not a marketing authorization and cannot be used to market Moderna commercially.
The FDA received Moderna’s application for EUA on April 26.
Based on clinical trials, Domingo said Moderna vaccine has an efficacy rate of over 94 percent in preventing COVID and over 80 percent efficacy among age groups above 18 years as well as special groups such as health care workers, people with comorbidities and the elderly.
Moderna is now widely used in the US and Europe.
Recorded adverse events following the use of the vaccine were mostly mild and transient, Domingo said.
He said vaccinees are closely monitored for possible allergic and adverse reactions.
“Since this is a new product, we are monitoring the vaccinees, who should report any adverse events following immunization or during use of the vaccine,” Domingo said.
At this time, Domingo said, all COVID vaccines in the country are under EUA, which is only valid during a public health emergency.
Only trained vaccinators will administer the vaccine and written consent must be taken from the vaccinee prior to vaccination.
More vaccines arriving
A total of 1.5 million doses from China’s Sinovac Biotech will arrive in the country tomorrow, Ambassador Chito Sta. Romana said.
Sta. Romana posted photos of the team from the Philippine embassy in Beijing – including First Secretary Winston Almeda, Economic Section Attaché Dada Aromin and Elizabeth del Mundo – inspecting boxes of COVID vaccines at the company’s warehouse in Daxing, Beijing on Tuesday.
The additional 1.5 million jabs will bring the total number of doses received from Sinovac to five million, a million of which were donated by China.
The Philippines has procured a total of 25 million vaccines from Sinovac.
Vaccine czar Carlito Galvez Jr. on Tuesday said that due to the massive spike in COVID cases in India – a major vaccine manufacturer – the Philippines may lower its vaccination target.
He said the 30 million shots the country ordered from India might be delayed as the Indian government prioritizes its people in administering locally made doses.
The Philippines aims to inoculate 70 percent of the country’s 110 million population to achieve herd immunity against COVID.
Meanwhile, Foreign Affairs Secretary Teodoro Locsin Jr. urged the public to pray for India’s recovery.
“She is and will always be the pharmacy of the planet. She’s just too way ahead. But she’ll have to create a more reliable supply chain for vital ingredients, all of which are mainly in the West because it is the most advanced in science,” Locsin said in a post on Twitter.
Sputnik doses
More than 2,000 individuals have received the first dose of Sputnik V vaccines in three cities in the National Capital Region (NCR), the Department of Health (DOH) said yesterday.
Health Undersecretary and national vaccine operation center chair Myrna Cabotaje said 2,634 doses of the 15,000 initial doses of Sputnik V that arrived in the country were administered in Parañaque, Manila and Makati on Tuesday.
Yesterday, she said the cities of Taguig and Muntinlupa started their inoculation using the vaccines from Russia’s Gamaleya Research Institute.
Cabotaje said local leaders in these cities are expected to finish the vaccination in five days.
The second dose will be administered 21 days after the first dose.
The 15,000 doses are part of 500,000 doses of Sputnik V vaccines procured by the government.
They were delivered in advance to be used to simulate the inoculation process as the Russian vaccines have different storage requirement than the Sinovac and AstraZeneca vaccines, which are already in the country.
Cabotaje said that as of May 4, there was no reported side effects of Sputnik V. – Helen Flores, Sheila Crisostomo
- Latest
- Trending


























