LGU vaccine deals show government not favoring any brand, Galvez says
MANILA, Philippines — The national government is not favoring any brand nor company in its national vaccination program, the chief implementer of the National Task Force against COVID-19 told members of the House on Monday.
To recall, senators noted over the weekend that the coronavirus vaccine developed by China-based drugmaker Sinovac may cost as little as $5, about P240, per dose, while estimated to cost $38, or more than P1,800, in the Philippines.
The Palace has said that Sinovac will be priced "not far from the price given to Indonesia, which is about P650 per dose."
RELATED: Cainta ends talks with Chinese vaccine supplier after uproar from residents
Speaking at the House committee on health briefing on the national government's vaccination program, vaccine czar Carlito Galvez Jr defended the government's choice to purchase 25 million doses of the Sinovac vaccine, pointing to the other companies being considered by local governments.
"This proves that we are not favoring any brand or country...we will have a fair mix of vaccine options. Only vaccines endorsed by the vaccine expert panel can be purchased. Only those issued with EUA will be administered by the DOH. With regard to the price, the negotiation will result in the best price available given our total volumes," Galvez said.
"We are now in the thick of negotiations and implementation. In the next few weeks we will be visiting the different mayors of Metro Manila...It is expected that we can start our rollout this first quarter in February," he also said, adding that the government is now working with 25 bilateral partners from ten different countries willing to conduct clinical trials and manufacturing.
RELATED: Claiming all jabs are the same, Duterte defends Sinovac purchase | Despite concerns on jabs' safety, Palace says public 'can't be picky' on vaccine preference
Galvez said at a Senate probe Friday that the government can still opt out of its deal for Sinovac vaccines, which have recorded a 50.4% efficacy rate overseas, if it so chooses.
"Centralized national procurement is the prevailing approach globally for both developed and developing markets," his presentation read.
Duque: Avoid privilege access to vaccines
The Duterte administration's national vaccination program seeks 148 million doses to inoculate 50-70 million Filipinos with coronavirus vaccines in 2021.
Asked about private sector participation in the program, Health Secretary Francisco Duque said that local governments and companies are still bound by the national prioritization program on vaccination.
"We have to avoid privilege access as a matter of policy," he said.
In late December, the Palace defended the advanced and smuggled vaccines taken by members of the Presidential Security Group, saying it was done to protect the president.
Presidential spokesperson Harry Roque later urged the public to "stop" discussing the incident, saying it was a closed issue.
The Washington Post reported over the weekend that there has been trade on the black market of COVID-19 vaccines. The Food and Drug Administration has so far been unsuccessful in catching people involved in the illegal trade of vaccines.
Ronald Mendoza, dean of the Ateneo School of Government, said in the report that the illegal PSG vaccination and the government's defense of using unauthorized vaccines "could encourage public reliance on the black market."
RELATED: Roque wants Filipinos to 'stop' discussion on illegal vaccines for PSG
How were the vaccines chosen?
Amid concerns about the efficacy of the Sinovac vaccine, infectious disease physician Dr. Edcel Salvaña told the House panel that vaccines like that of Sinovac would at the very least prevent severe cases of the virus.
Conclusive figures on the efficacy of Sinovac's vaccine remain elusive and unclear to date, though clinical trials conducted in Brazil yielded 50.4% efficacy compared to other jabs including that of Pfizer at 95% and AstraZeneca at 70%.
"This is why we need to use all available vaccines right now to prevent the risk, because they all prevent severe infection," he said.
The Department of Health's vaccine experts panel also presented the priorities of its vaccine matrix, namely:
- Vaccines in advanced stages of development (Phase III)
- Track record of the company in vaccine development
- Technology platform and vaccine storage
- Safety profile and adverse events reported in first two phases
- Efficacy and immune correlates of protection (neutralizing antibodies data)
- Existing emergency use authorization from other countries
Food and Drug Administration Director General Eric Domingo said that other companies have also signified their intention to apply for EUA.
The following companies have already applied for EUAs in the Philippines, he said:
- Pfizer-Biontech: Applied December 23, 2020
- AstraZeneca: Applied January 6, 2021
- Gamaleya-Sputnik V: Applied January 7, 2021
- Sinovac: Applied January 13, 2021 (Pre-assessment)
Why does this matter?
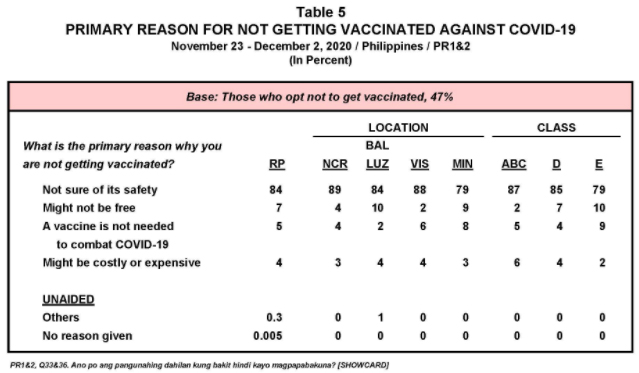
- Survey results by the Social Weather Stations suggest that some 47% of the public would not be willing to be vaccinated due to concerns about the safety of the jabs.
- In response to the unflattering sentiment, the Palace insisted there would be no other choice.
- As of the Department of Health's latest case bulletin Sunday, the national caseload in the Philippines has breached 500,000 coronavirus cases. Exactly 24,691 of these are still active cases.
- Over the past few weeks, local chief executives across the country have already signaled their intent to secure vaccination for their constituents ahead of the rollout of the mass vaccination program by the national government.
RELATED: A roundup of local governments who secured vaccine deals in advance
“Bukod sa 47 percent na ayaw magpabakuna, meron pang 27 percent na di siguradong magpapabakuna. Kaya kailangan natin ang pinakaligtas at pinakamabisang bakuna para sa ating mga kababayan. Hindi pwedeng ‘pwede na’,” Pangilinan said.
(Apart from the 47 percent who do not want to be vaccinated, there are also 27 percent who are not sure about getting vaccinated. This is why we need the safest and most effective vaccine for our countrymen. We can't settle for 'good enough.')
- Administration officials continue to defend the government's choice to purchase the vaccine of Sinovac Biotech Ltd., a privately owned corporation based in Beijing, China, for its national inoculation program amid mounting concerns about the vaccine’s price and efficacy.
“Sinovac has a track record of bribery, yet why insist on dealing with them?" Lacson asked in an interview aired over DWIZ on Saturday.
"Considering all these, can we blame the lawmakers and even our countrymen why they express suspicion in the government’s vaccination program?" he added.
— with reports from Bella Perez-Rubio and Christian Deiparine
- Latest
- Trending






























