Palace taps team of experts to probe Dengvaxia controversy

MANILA, Philippines — Malacañang is forming a three-man team of foreign health experts to conduct independent scientific research on the impact of Dengvaxia immunization in the country.
Presidential spokesman Harry Roque Jr. yesterday said President Duterte wants a more comprehensive and independent report after the Public Attorney’s Office (PAO) and the Department of Health (DOH) seemed to have conflicting reports on the matter.
“The President, after much discussion, said that he will create a three-man panel of experts; all Asians, no Westerners and he will be bound by the findings of this three-man expert team on the issue of whether or not Dengvaxia actually caused deaths,” he said.
“Now the problem of the President is – although there is a finding of a PAO expert and there is the Philippine General Hospital panel of experts findings – as a lawyer and a former prosecutor, he knows that expert witnesses can cancel out each other’s testimonies,” he added.
Roque said the sound opinion from independent experts will help in the probe to strengthen the government’s move to make pharmaceutical firm Sanofi Pasteur, the manufacturer of Dengvaxia, accountable for the mess.
A list of expert pathologists submitted by the DOH is now with the President.
The Palace is also hoping that Congress will pass a bill allowing the use of the P1.16 billion refunded by Sanofi to help those who received Dengvaxia.
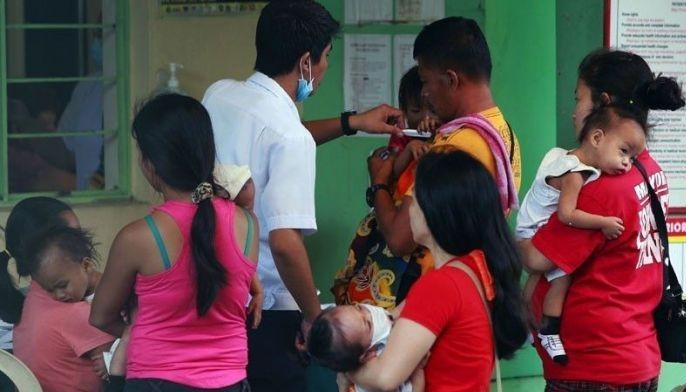
The DOH suspended the mass immunization program on Dec. 1, 2017, a day after Sanofi Pasteur disclosed that Dengvaxia poses threat of severe symptoms to those who had not been previously afflicted with dengue.
The vaccines were administered to about 800,000 public school students aged nine years old and above starting April 2016.
Several groups, including Gabriela, alleged that former health secretary Janette Garin, former president Benigno Aquino III and former executive secretary Paquito Ochoa conspired with officials of Sanofi in purchasing the vaccine in bulk in 2015 even when there was no comprehensive study yet on its efficacy and risks.
Gabriela last year filed a graft complaint against Garin, Aquino and Ochoa before the Office of Ombudsman in connection with the controversy.
COA questions PCMC’s Dengvaxia procurement
Amid the ongoing investigation of health experts and PAO, the Commission on Audit (COA) reported that the Philippine Children’s Medical Center (PCMC) also committed procedural lapses when it procured P3 billion worth of Dengvaxia in 2016.
In its 2017 audit report, COA said the procurement “was not in accordance with the Implementing Rules and Regulations (IRR) of Executive Order No.49, the Revised Implementing Rules and Regulations of the Government Procurement Reform Act and the Memorandum of Agreement by and between the DOH and PCMC.
But the COA has admitted that its audit findings and recommendations have become “moot and academic.”
The commission explained it included this observation in its latest annual audit report to “ensure that the implementation of future projects, programs and transactions of similar nature be dealt with proper caution and due observance of laws, rules and regulations governing the transactions.”
Garin earlier stated during a Senate hearing that its was the PCMC which was tasked to purchase Dengvaxia from multinational pharmaceutical company Sanofi Pasteur as part of the implementation of the Aquino administration’s P3.5-billion dengue immunization program launched in 2015.
The COA, in its audit report, identified four “deficiencies” in the PCMC’s procurement of the dengue vaccines.
The COA said the PCMC violated DOH Administrative Order No. 2012-0023 which serves as the IRR for Executive Order 49.
Under EO 49, only the drugs included in the Philippine National Drug Formulary (PNDF) manual should be purchased by the government.
The COA said that while the IRR of EO 49 states that exemption from the PNDF manual may be allowed, it is subject to approval of the program director of National Center for Pharmaceutical Access and Management (NCPAM) upon recommendation of Formulary Executive Council (FEC) “based on prescribed criteria.”
The COA pointed out that this protocol was not followed by PCMC when it procured Dengvaxia.
“Review of the documents submitted showed that the FEC recommendation for provisional exemption from PNDF was not submitted to the NCPAM Program Director as required under DOH AO No. 2012-0023 but to the DOH secretary,” the COA report read.
Second, the COA said purchase of the vaccines violated Section 39 of the revised IRR of the Government Procurement Reform Act, which requires the winning bidder to issue a performance security prior to the signing of contract with the government.
Performance security serves as a bond or guarantee from a supplier to ensure faithful compliance with what was agreed upon in the contract.
The COA said its audit review revealed that Sanofi issued its performance security in the form of Irrevocable Domestic Standby Letter of Credit (LC) only on March 10, 2016 or a day after the effectivity of the PCMC’s purchase order.
Furthermore, the COA said the LC’s expiry was dated Sept. 6, 2016, five months ahead of the last delivery of the procured vaccines in February 2017 as per the contract’s terms of reference (TOR).
Third, the COA said the shelf life of the vaccines as indicated in the approved purchase order was only for 12 months when the TOR required that the dengue vaccine should have a shelf life of 18-24 months upon delivery.
Lastly, the COA said the PCMC already conducted pre-procurement activities as early as January 2016 even before the MOA between the DOH and PCMC took effect on Feb. 19, 2016.
PCMC comment
In its comment incorporated in the audit report, the PCMC management argued that “the issue on the exemption of the dengue vaccine from the requirements of EO 49 (was) already beyond PCMC” after Garin, as then DOH secretary, issued the certification of exemption.
The PCMC management also said “it is not true that bidding was conducted before the effectivity of the MOA.”
The PCMC pointed out that the DOH legal service reviewed the MOA on Jan. 14, 2016 and posted no objection to it, thus it was considered effective from then and the Feb. 19 date was “only the date of notarization.”
With regard to Sanofi’s alleged delayed posting of its performance security, the PCMC explained that while the purchase order was dated March 9, 2016, it was signed by the representatives of Sanofi only on March 10 as shown in the certification at the right bottom portion of the PO.
The PCMC further argued that “any issue on the posting of performance security has become moot and academic after the completion of delivery of dengue vaccine on Feb. 28, 2017.”
Lastly, the PCMC said the issue on the 12-month shelf life of the vaccines as stated in the PO is
a “mere inadvertence.” The PCMC said there was an amendment attached to the PO stating that it shall adopt all the terms under the TOR, including the requirement that the vaccines to be delivered shall have a shelf life of 18 to 24 months.
De Lima questions Gordon’s report
Last month, Sen. Richard Gordon released the Senate Blue Ribbon committee report recommending the filing of charges against Aquino and his former officials in connection with the
Dengvaxia controversy.
The report claimed the supposed haste that allegedly characterized the procurement of the vaccine was caused by a premeditated scheme hatched by the Aquino administration to use the public immunization program as a campaign tool for the Liberal Party’s presidential election campaign in 2016.
Opposition Sen. Leila de Lima has questioned Gordon’s report, citing what she said were serious flaws.
De Lima said the report arbitrarily cut out relevant events in the timeline and distorted facts, which were clearly and categorically established during the hearings in the Senate, to demonize Aquino.
“The report made it out to appear that former president Aquino and former secretary Garin knowingly bought poison for injection into the bodies of Filipino children, just short of the genocide accusation hurled at them by some quarters,” she said in her dissenting report on the Dengvaxia probe.
“The chairman (Gordon) may not believe it, but there was actually reasonable ground for the government to act as it did in the procurement of the dengue vaccine. The vaccine after all was approved for use by the national regulatory agencies of 20 countries,” she added.
Contrary to Gordon’s report, De Lima noted that the World Health Organization Strategic Advisory Group of Experts eventually came out with a position paper that did not prohibit the use of Dengvaxia, even as it noted that it can be considered for use in countries and areas where the dengue virus was endemic, like the Philippines.
“What is clear is that Dengvaxia has benefits as well as risks, and the reason why the experts, including WHO, considered the dengue vaccine for use in countries with high seroprevalence is because they concluded, based on the clinical trials of Sanofi-Pasteur, that its benefits outweighed the risks,” she said.
The discussion about the dengue vaccine started during the time of health secretary Enrique Ona, to his successor Garin and ended in the time of her successor, Paulyn Ubial.
De Lima criticized the report for making former Ona’s resignation appear to be a mystery, thereby insinuating that he was forced to eventually resign because he was against Aquino’s supposed scheme to use the dengue vaccine for the May 2016 elections.
“This insinuation is repeated several times in the report, even when the records of the hearing are clear on secretary Ona’s categorical denial of this malicious account of the reasons behind his resignation,” she said.
“Gordon was in a one-on-one cross-examination with the witness. The witness categorically told him that his resignation has nothing to do with either Sanofi or his refusal to go on board the procurement of Dengvaxia,” she added. – With Elizabeth Marcelo, Paolo Romero
- Latest
- Trending